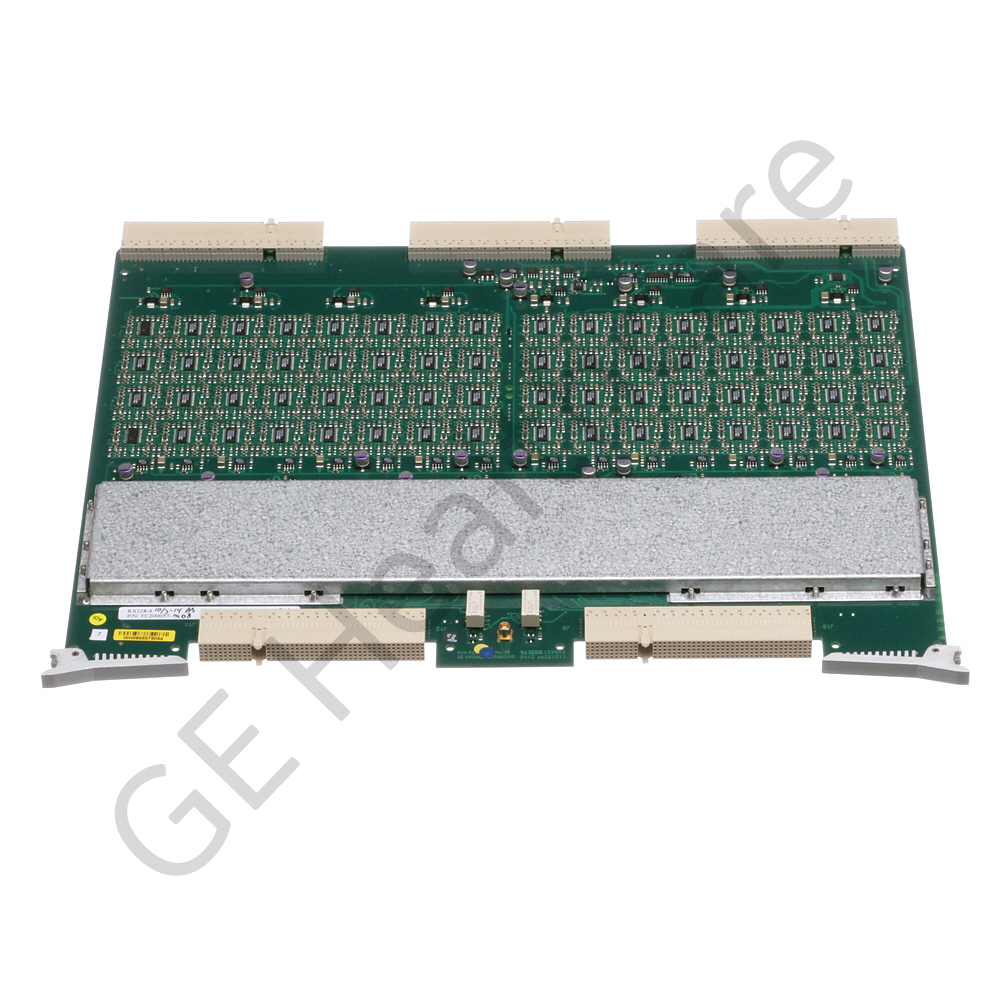

RX 128 BOARD VER. 4
| FC200057-R | |
| Ultrasound | |
| GE HealthCare | |
| GE HealthCare | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Product Overview
- The RX 128 BOARD VER. 4 in Ultrasound is used in Vivid 7. The board offers reliable operation, efficient functioning and longer life time. They are compact and can be easily portable. It is made from the material which has combination of 55% aluminum, 43.4% zinc and 1.6% Silicone. The products superior material quality offers Excellent corrosion resistance, easy processing, improves formability, heat reflectivity and has a shiny sparkling appearance. It is tested, affordable and compatible to use. The GE product is an Innovation and technology which fits well into versatile customer needs. The part is diligently designed for high Performance and reliability. It is securely packaged inside a High-quality packing box to avoid physical damage during transit and labeled with details about the product, Quality Assurance (QA) seal and shipment details. They are also used in rack Assay BT03 V/RFI, vantage upgrade, vivid 7 rack assay, BT05, V7 UPGR. BT02-BT03 HW & SW and other medical equipments as applicable.