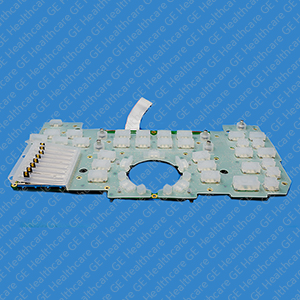

Lower Switch Board with Elastomer
| GA200440-R | |
| Ultrasound | |
| GE HealthCare | |
| GE HealthCare | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Product Overview
The Lower Switch Board with Elastomer is comprised of a lower switch board and silicone elastomer with integrated slide pot filter area. The switch board has a ribbon cable for connecting to the main controller board. Elastomer switches or rubber keypads are manufactured from silicone rubber properties that are typically processed from injection molding process. The Injection molding manufacturing process helps to achieve good product consistency. They have exceptional resistance to extreme temperatures and environmental factors making them an ideal choice for products exposed to harsh environmental conditions, moisture and chemicals. Elastomer silicone rubber keypads offer a wide range of design possibilities due to the many sizes, shapes, and colors. It is packed per the packaging requirement procedure so that the minimum protection required for safe delivery to a destination is based on each unit’s physical characteristics. It is a spare part, which can be replaced whenever required. It is specially designed for the UL Vivid E9 operator panel.