CCA, DCB, MAESTRO LU41600-R
| LU41600-R | |
| Bone Health | |
| GE HealthCare Parts | |
| GE HealthCare | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Product Overview
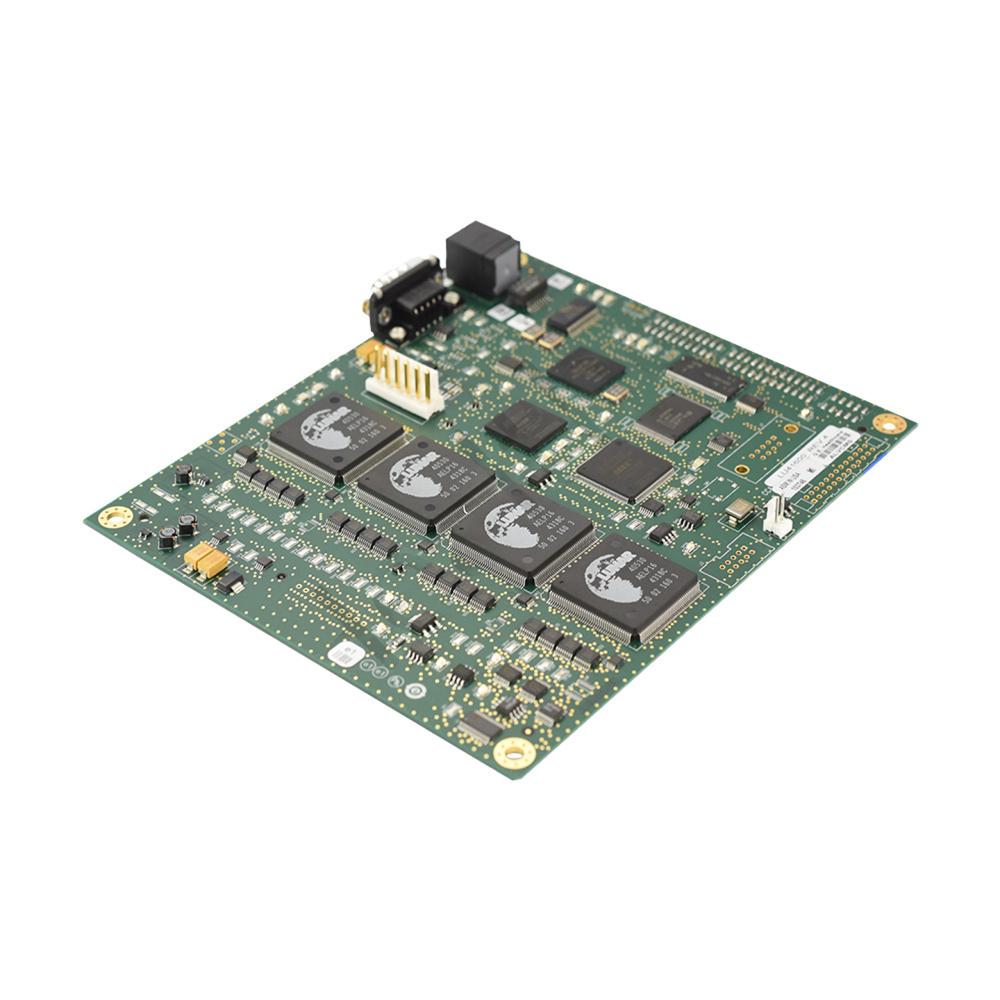
The Circuit Card Assembly (CCA) DAS Control Board is the detector control board (DCB) of the Maestro bone densitometer. This circuit card assembly is an embedded controller of the Maestro system. It is responsible for all Maestro detector control and support operations. It is intended to run as a master of the Maestro XMS CCA and slave to the host PC. This DCB is designed to take advantage of many of the on-chip modules of the MDF5280. The Detector Control Board (DCB) has many built-in features such as Inter-integrated circuit (I2C) bus controller, programmable software watchdog time, synchronous DRAM controller for two banks of SDRAM memory and up to seven chip-select signals. This DCB provides the enhanced diagnostic capability. This diagnostic tool allows service to distinguish detector failures from electronics failures (if test passes, electronics from eV ASIC are considered good). A second diagnostic circuit on board the DCB allows firmware to disconnect detector inputs ahead of the bipolar shaping stage and inject a test pulse on multiple channels. This diagnostic allows service to distinguish between detector and DCB failures. This assembly is compatible with GE's Lunar iDXA 2 system. The assembly will be shipped in suitable packaging to give adequate protection during transit.
LU41600-R